Stim2Go

Stim2Go
Wearable App-Controlled Neurorehabilitation System for Non-Invasive Electrical Stimulation and Biofeedback
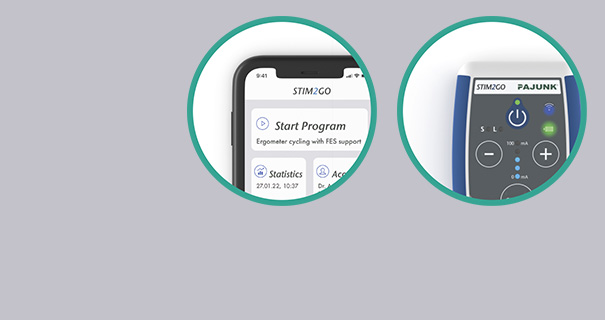
Stim2Go is a powerful neurostimulation system developed for non-invasive muscle re-education by use of Functional Electrical Stimulation (FES) and Neuromuscular Electrical Stimulation (NMES) as well as pain via Transcutaneous Electrical Nerve Stimulation (TENS). Stim2Go uses a built-in sensor to capture the patient's motion in real time. With this biofeedback, stimulation patterns can be triggered to support the patient in its movement execution. Stim2Go is app-controlled by a smart device – the app is freely available in the Apple App Store / Google Play Store.
Stim2Go Overview
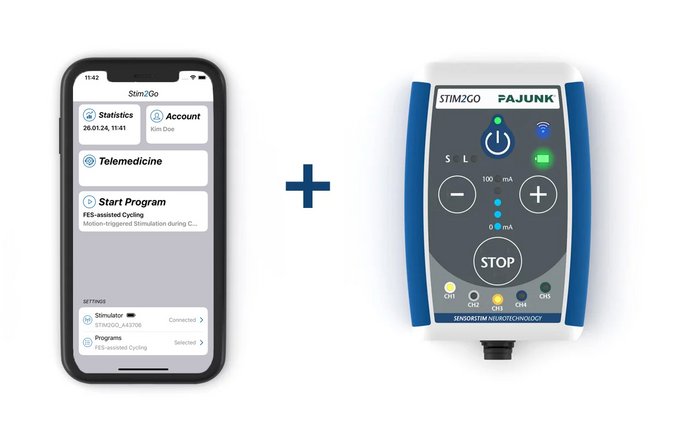
Stim2Go Features & Advantages

Wearable Device
- The compact and light weight design allows the device to be easily worn during mobility activities

Intuitive App Design
- Modern, easy to use, intuitive smart device app interface for professionals and patients

5 Stimulation Channels
- Up to 5 stimulation channels can be used independently
Powerful, Long Pulse Duration
- Pulse duration can be adjusted by the therapist
- For high BMI
- For reaching deeper neural structures

Lumbar Stimulation
- Relearn voluntary motor functions, relaxation of muscle spasms, prevention or retardation of disuse atrophy & muscle re-education (as FES/NMES)
- Pain management (as TENS)
- Long pulse duration

FES-Assisted Cycling
- Motion-triggered by body-worn stimulator (Biofeedback)
- Use of existing cycling equipment without any modifications

Compliant Skin Electrodes
- ValuTrode® cloth electrodes optimally support Stim2Go therapies
- Wizard for electrode placement and selection of size & shape
- Superior quality at an affordable price
- Durable and multiple applications for the skin

Augmented Reality (AR)
- AR support for electrode placement

Custom Programs
- Customizable and individually assignable programs for patients
- Frequency: 1-100 Hz
- Current amplitude: max 100mA @ 1kΩ
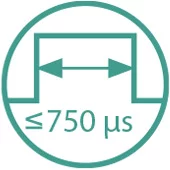
- Pulse-width: up to 750 µs
- Pulse form: bi-phasic rectangular (charge-balanced)
- APP-based program storage for device independency

Interactivity
- Group therapy and telemedicine features
- Assignment of programs by therapists for patients
- Account types with different editing and customization rights and features

Clinic Features (Collaboration License Required)
- Shared customized programs and patients’ statistics in groups of therapists within a facility
Indications for Use
As a powered muscle stimulator (NMES) the Stim2Go is indicated for the following conditions:
- Relaxation of muscle spasm
- Prevention or retardation of disuse atrophy
- Increasing local blood circulation
- Muscle re-education
- Immediate post-surgical stimulation of calf muscles to prevent venous thrombosis
- Maintaining or increasing range of motion
As a transcutaneous electrical nerve stimulator (TENS) for pain relief the Stim2Go is indicated for the following conditions:
- Symptomatic relief and management of chronic (long-term), intractable pain
- Adjunctive treatment in the management of post-surgical pain and post traumatic acute pain
As a biofeedback device the Stim2Go is indicated for the following condition:
- Muscle re-education purposes
As a functional neuromuscular stimulator, the Stim2Go is indicated for the following conditions:
- Helps to relearn voluntary motor functions of the extremities
The movement-based biofeedback can only be used for patients with an active range of motion (ROM) which is defined as the ability of volitional movement resulting in a change of the concerned limb inclination angle of at least 10 degrees.
Programs
STIM2GO Ordering Info
| Item description | Item no. | Purchase Unit |
|---|---|---|
| Complete set incl. stimulator, 2 & 5 channel cable, adapter cable, strap, strap holder, power supply with 3 adapters (EU, US, UK) | 9151-10-00 | 1 |
| Item description | Complete set incl. stimulator, 2 & 5 channel cable, adapter cable, strap, strap holder, power supply with 3 adapters (EU, US, UK) | |
|---|---|---|
| Item no. | 9151-10-00 | 1 |
| Item description | Item no. | Purchase Unit |
|---|---|---|
| Power supply with 3 adapters (EU, US, UK) | 9151-00-10 | 1 |
| 2-channel cable | 9151-01-11 | 1 |
| 5-channel cable | 9151-01-12 | 1 |
| Adapter cable 1:2 | 9151-01-13 | 1 |
| Charging cable | 9151-01-14 | 1 |
| Strap and strap holder | 9151-02-00 | 1 |
| Item description | Power supply with 3 adapters (EU, US, UK) | |
|---|---|---|
| Item no. | 9151-00-10 | 1 |
| Item description | 2-channel cable | |
| Item no. | 9151-01-11 | 1 |
| Item description | 5-channel cable | |
| Item no. | 9151-01-12 | 1 |
| Item description | Adapter cable 1:2 | |
| Item no. | 9151-01-13 | 1 |
| Item description | Charging cable | |
| Item no. | 9151-01-14 | 1 |
| Item description | Strap and strap holder | |
| Item no. | 9151-02-00 | 1 |
| Item description | Item no. | Purchase Unit |
|---|---|---|
| ValueTrode 3 cm (round) – 1 bag with 4 electrodes | 9151-05-01 | 1 |
| ValueTrode 3 cm (round) – 10 bags with 4 electrodes | 9151-06-01 | 10 |
| ValueTrode 4 x 6 cm (oval) – 1 bag with 4 electrodes | 9151-05-02 | 1 |
| ValueTrode 4 x 6 cm (oval) – 10 bags with 4 electrodes | 9151-06-02 | 10 |
| ValueTrode 5 x 5 cm (square) – 1 bag with 4 electrodes | 9151-05-03 | 1 |
| ValueTrode 5 x 5 cm (square) – 10 bags with 4 electrodes | 9151-06-03 | 10 |
| ValueTrode 5 x 9 cm (rectangle) – 1 bag with 4 electrodes | 9151-05-04 | 1 |
| ValueTrode 5 x 9 cm (rectangle) – 10 bags with 4 electrodes | 9151-06-04 | 10 |
| Item description | ValueTrode 3 cm (round) – 1 bag with 4 electrodes | |
|---|---|---|
| Item no. | 9151-05-01 | 1 |
| Item description | ValueTrode 3 cm (round) – 10 bags with 4 electrodes | |
| Item no. | 9151-06-01 | 10 |
| Item description | ValueTrode 4 x 6 cm (oval) – 1 bag with 4 electrodes | |
| Item no. | 9151-05-02 | 1 |
| Item description | ValueTrode 4 x 6 cm (oval) – 10 bags with 4 electrodes | |
| Item no. | 9151-06-02 | 10 |
| Item description | ValueTrode 5 x 5 cm (square) – 1 bag with 4 electrodes | |
| Item no. | 9151-05-03 | 1 |
| Item description | ValueTrode 5 x 5 cm (square) – 10 bags with 4 electrodes | |
| Item no. | 9151-06-03 | 10 |
| Item description | ValueTrode 5 x 9 cm (rectangle) – 1 bag with 4 electrodes | |
| Item no. | 9151-05-04 | 1 |
| Item description | ValueTrode 5 x 9 cm (rectangle) – 10 bags with 4 electrodes | |
| Item no. | 9151-06-04 | 10 |
| Item description | Item no. LUER | Purchase Unit |
|---|---|---|
| Collaboration license - groups up to 10 therapists | 9151-050015 | 1 |
| Extention license - 5 additional therapists | 9151-050016 | 1 |
| Item description | Collaboration license - groups up to 10 therapists | |
|---|---|---|
| Item no. LUER | 9151-050015 | 1 |
| Item description | Extention license - 5 additional therapists | |
| Item no. LUER | 9151-050016 | 1 |
Stim2Go has been cleared for market by the FDA. It cannot be made available in other countries until it is CE marked according to Regulation (EU) 2017/745.