
PAJUNK®
SINCE 1965
Looking back and forward to the future.


TRUST TRADITION. EXPERIENCE INNOVATION
PAJUNK® has stood for innovative medical technology made in Germany for 60 years.
Today Pajunk GmbH is one of the global market leaders in the field of regional anesthesia. It is a world leader particularly in spinal anesthesia, epidural anesthesia, peripheral nerve block techniques and ultrasound-guided nerve blocks. At the same time, we have been a guarantor of quality, reliability and innovation for our customers in the field of anesthesia for decades.
With the regulatory changes currently prevailing in the medical device market (MDR) and the major shifts on the market, especially in the regional anesthesia segment (refocusing, sale of former competitors), as well as the introduction of new standards (NRFit, ISO 80369-6), Pajunk remains one of the few mainstays on which our customers and patients can depend as a reliable partner.
Pajunk GmbH aims to build on this leading position and status in the field of regional anesthesia, and at the same time establish further footholds in pain therapy and neurology/neurological rehabilitation.
Headquartered in Geisingen near Tuttlingen in southern Germany, a region also known as the “world center of medical technology”, Pajunk operates worldwide. We now have subsidiaries in Germany (also based in Geisingen) with sales offices for the whole of Central Europe, the UK, Switzerland and the USA. All other countries are served by long-standing distribution partners.
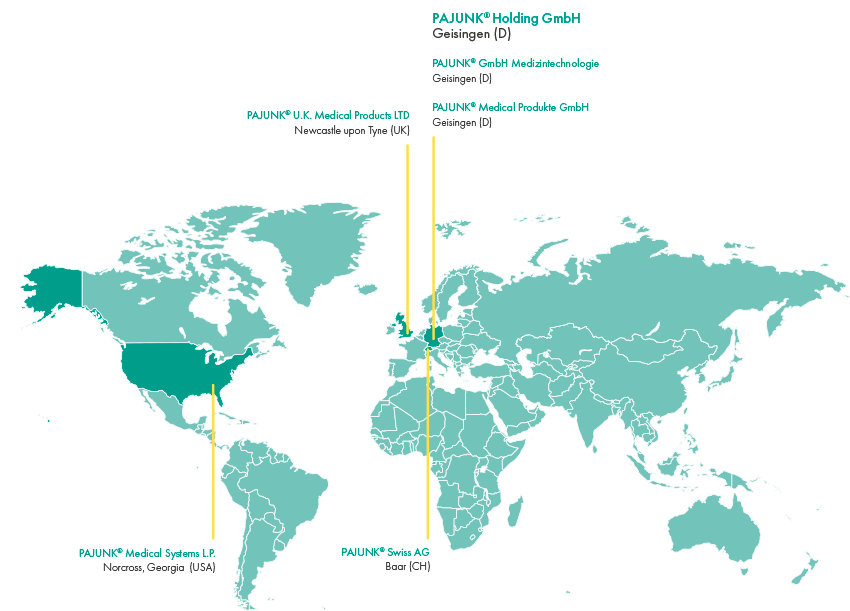



The company can look back on 60 years of success and is now a medium-sized family business with around 650 employees. It is managed in the second generation by Simone Pajunk-Schelling and Martin Hauger. The company was founded in 1965 as a start-up in a family garage in Geisingen by the brothers Horst and Heinrich Pajunk. Since then, it has grown continuously and the Geisingen site expanded regularly.

OUR FORMULA FOR SUCCESS.
The secret of our success has always been our close cooperation with the medical professionals who have approached Pajunk with product ideas and continue to do so today. This is how our most important products have been created - with real added value and unique competitive advantages for the user. Pajunk currently holds 14 patents and 18 registered trademarks.
Here are a few groundbreaking examples:
Sprotte®

With the introduction of the first atraumatic Sprotte needle at the end of the 1970s together with Professor Sprotte, spinal anesthesia was restored as a fully accepted alternative to general anesthesia. A unique tip design is the secret of its success. Sprotte’s atraumatic puncture and optimal gliding properties minimize possible injuries and prevent foreign bodies and tissue particles from being carried into the subarachnoid space, while reducing the incidence of post-dural puncture headache (PDPH). More about Sprotte®
SonoPlex®

In collaboration with Dr. Chris Mitchell, we have succeeded in creating an effect that allows the ultrasound to always return to the transducer, thus generating the best possible ultrasound image. SonoPlex® - launched in 2008 - has revolutionized the Ultrasound Guided Puncture and is one of our most important product families today. More about SonoPlex®
“It was an amazing experience to join up with Pajunk, to work so much as a team and nearly family, to get this product onto the market as quickly as possible. And literally from the time I met them in person to the time the product was on the market was only 12 months.”
Dr. Chris Mitchell

E-Cath®
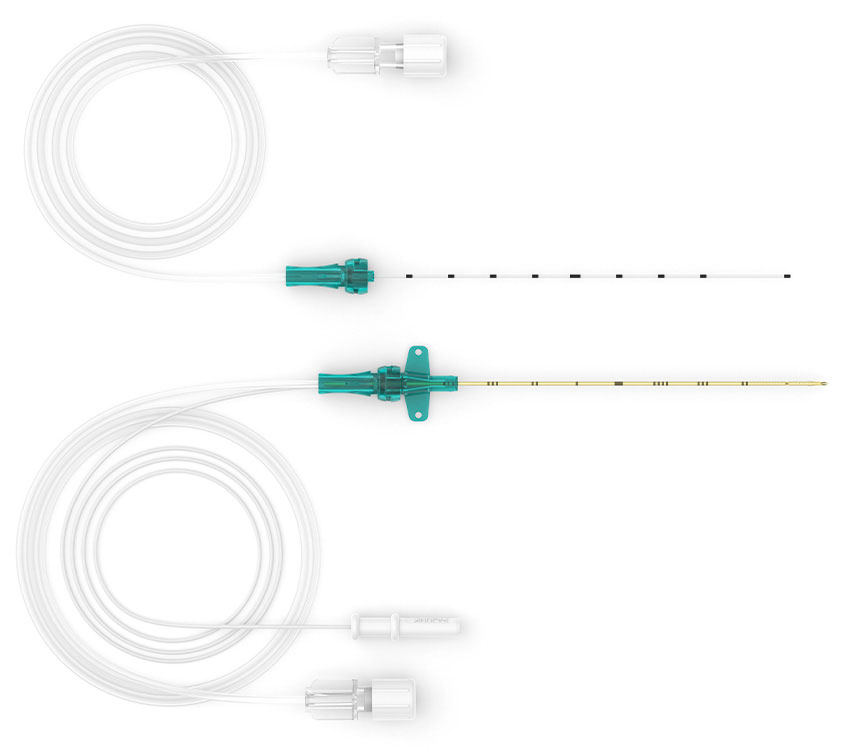
Developed together with Dr. Ban Tsui, the E-Cath® II / E-Cath® II Plus - launched in 2024 - combines the best from clinical experience and engineering expertise, resulting in a system that addresses the biggest catheter problems in Continous Nerveblocks: complexity, catheter kinking, anaesthetic leakage and catheter dislocation and migration. More about E-Cath®
TRUST IN QUALITY.
All feedback we receive from the market is examined closely, and incorporated directly and quickly into the further improvement of our products in our development department.
In general, Pajunk stands for precision down to the smallest detail. Good is not good enough for us. As specialists, we want to be one of the best. We apply our in-depth expertise gained from decades of research to our latest technological applications. Our state-of-the-art production facilities cover the full range of processing steps required in our business. In addition, we are able to develop new products independently and integrate them quickly into our existing processes. We set the highest quality and production standards in both raw material sourcing and manufacturing. Strict quality management from development through all production stages to the final product, with monitored cleaning and control procedures, guarantees maximum process reliability.


In particular, our high-quality clean rooms (ISO 14644-1 Class 7 and EU GMP Class D) enable us to manufacture sterile products in Geisingen to a high standard.


As an international company, we meet the increasingly stringent requirements for global product approvals, ranging from development documentation to clinical evaluation and risk management. Our headquarters in Geisingen, Germany, are located in the heart of the German medical technology industry, giving us access to a highly skilled workforce that we train and develop continually.
SUSTAINABILITY IS CLOSE TO OUR HEART.
Sustainability in all its facets has always been close to Pajunk’s heart. Not only in the area of environmental awareness, but also in social commitment and responsible corporate governance. For example, we are striving to achieve CO2 climate neutrality, to uphold high values of social commitment and to act sustainably as a company in order to be able to offer added value to future generations. Learn more about our sustainability roadmap here.


